Arrhenius equation: Increasing temperature increases the rate reaction
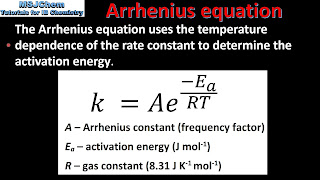
The rate of reaction of a particular reaction often increases as one increase the temperature. However, how can this belief be proved using Arrhenius equation? According to the Arrhenius equation, the rate constant (k) is obtained by the ratio of Ea to RT, in which Ea denotes required energy while RT is the available thermal energy. The Arrhenius Equation is given by:
k=Ae-(Ea/RT)
When Ea is larger, it
results in slower reactions. On the other hand, when RT is large, it leads to
faster rate of reaction. Larger Ea gives smaller rate constant k, but larger RT
produces larger rate constant (k). Larger rate constant increases the rate of
reactions.
By using Arrhenius
equation, one can easily see that when the temperature is increased, the rate
constant will also increase.
Let us consider these
two scenarios. In the first scenario, Ea=120,000 Joules/mole, R=8.31 mol-1
K-1, and K=373K.In the second scenario, Ea=120,000Joules/mole,
R=8.31 mol-1 K-1, and K=423K.
Substituting these
numbers into Arrhenius equation when keeping A and k values constant gives a
fraction of collision as follows:
Fraction of collisions=e-(120,000Joules/mole)/ (273K x 8.31 mol-1 K-1)…………………………Scenario 1
Fraction of collisions=e-(120,000Joules/mole)/ (273K x 8.31 mol-1 K-1)…………………………Scenario 1
Fraction= e-(38.7)
= 1 x 10-17
Fractions of
collisions= e-(120,000Joules/mole)/ (423K x 8.31mol-1K-1) ……………………...Scenario
2
= 1.5 X 10-15
In scenarios 1 and two
show that increasing temperature of the reaction from 373K to 423K increases
fraction of collisions from 1 x 10-17 to 1.5 X 10-15. Therefore,
increasing temperature increases the number of useful collisions of components
participating in a chemical reaction.



The Most Iconic Video Slots On The Planet - Jancasino
ReplyDeleteThe most iconic worrione.com video slot is the 7,800-calibre slot machine called Sweet Bonanza. This slot machine was developed 바카라 사이트 in jancasino 2011, developed https://shootercasino.com/merit-casino/ in the same aprcasino.com studio by
UcapvoOquiyu1993 Timothy Wilson https://marketplace.visualstudio.com/items?itemName=1sugacaze.Descargar-Sheltered-gratuita
ReplyDeleteatneystinem