Sample of chemistry lab report on activation energy of reactions
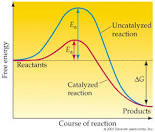
The activation energy of reaction is the minimum energy needed to initiate a chemical reaction and it is usually designated by E a . Its SI unit is either in Kilocalories/ mole (Kcal/mole) or KJ/Mole (Kilojoules/mole). A chemical reaction proceeds at an appreciable rate when transitional energy and a good number of molecules present,which are the same or greater than activation energy. In this experiment, the activation of energy for the reaction below was determined: The data of rate equation (rate = K [I - ][BrO - ][H+] 2 ) for this reaction versus temperature (°C) matched Arrhenius equation and gave the approximate activation energy. In K (T) = -Ea/R + (1/T) + InA (Slope-intercept form) Or InK (T2) - InK (T1) = -E a /R (1/T2 -1/T1), (point-slope form). According to Arrhenius Equation, plotting InK versus (1/T) possess a slope (m),which is equivalent to -E a /R. R= 8.314J/ (Kmole). The activation energy of the reaction was