Biochemical process of light independent stage of photosynthesis
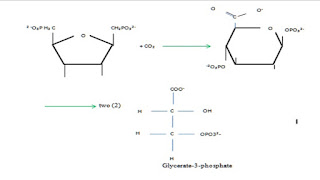
In this stage carbon (IV) dioxide from atmosphere is captured and modified by addition of hydrogen to form carbohydrates and other organic compounds (amino acids and lipids). Carbon (IV) dioxide combines with ribulose-1, 5-bisphosphate to form unstable six carbons sugar which eventually breaks down to two (2) molecules of glycerate-3-phospahte, check it here; Two molecules of glycerate-3-phospate are phosphorylated by ATP to form two (2) molecules of glycerate disphosphate, NADPH catalyzes them, reducing them to two molecules glyceraladehyde-3-phospate. One molecule of glyceraldehyde-3-phosphate is quickly converted to glucose, amino acids, lipids and other carbohydrates while the other molecule is utilized in the synthesis of ribulose-1,5-bisphospahte. This process is as follow; In this process, first stable molecule is glycerate-3-phosphate . Energy carriers (ATP and NADPH) are used to reduce glycerate-3-phosphate to form 12 mo...
