Sample of chemistry lab report on activation energy of reactions
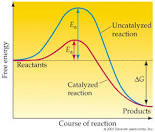
The activation energy of reaction is the minimum energy needed to initiate a chemical reaction and it is usually designated by Ea. Its SI unit is either in Kilocalories/ mole (Kcal/mole) or KJ/Mole (Kilojoules/mole). A chemical reaction proceeds at an appreciable rate when transitional energy and a good number of molecules present,which are the same or greater than activation energy. In this experiment, the activation of energy for the reaction below was determined:
In K (T) = -Ea/R + (1/T) + InA (Slope-intercept form)
Or
InK (T2) - InK (T1) = -Ea/R (1/T2 -1/T1),
(point-slope form).
According to Arrhenius Equation, plotting InK versus
(1/T) possess a slope (m),which is equivalent to -Ea/R. R= 8.314J/ (Kmole). The activation energy of the reaction was determined by
running the reaction at different temperatures (23.7°C, 31.5°C, 43.5°C and 10.2°C).
Procedure
The hot plate was plugged, and water heated to a temperature
of 20-25°C on low setting. 250ml of tap water was added into a 400ml beaker and
set on the hot plate. Test tubes A and B, which contained appropriate mixtures
were prepared and allowed to reach a proper temperature, while swirling a bit
for every 3 minutes to ensure consistency of temperature within the test tubes.
Data table was prepared for four runs at different temperatures. When
temperature stabilized, the stopwatch was prepared and test tubes were removed
from the water bath. Solution B was quickly added to solution A while starting
the stopwatch immediately as they were mixing, then placing the test tube of the
mixture back into the water bath. A Stopwatch was stopped when the familiar color
(blue-black) of the reaction was observed and digital thermometer used to
determine the temperature of the mixture when the reaction reached completion.
The temperature and the time were recorded in the table, and the solutions disposed off
in waste bucket.
Fresh solutions, A and B,
were prepared as earlier and by adjusting the hot plate to temperature of 30-35°C
and repeating process, starting from the time the stopwatch was employed in the
process until disposal of the solutions. The same was done for temperature
40-45°C.
The hot plate was
unplugged, followed by pouring out warm water from last trial and replacing it
with 250ml cold tap water. Ice was added to the water to reduce its temperature
to -10°C, but ice was removed when temperature dropped below -10°C. Two fresh
solutions of A and B were prepared and placed in the beaker as the water was
cooling down and earlier steps in paragraph one under procedure were repeated
when solution cooled to desired temperature (-10°C).
Results
The following results were
obtained:
Calculation
The calculations for rate of reactions at different
temperature were as indicated below:
Rate = (3.33 X 10-5 M)/dt
For run # 1:
= (3.33 X 10-5M)/
(112 -0) s
= 2.97 X 10-7M/s
For run #2:
= (3.33 X 10-5M)/
(67-0) s
= 4.97 X 10-7M/s
For run 3:
= (3.33 X 10-5M)/
(36-0) s
= 9.25 X 10-7M/s
For run #4:
= (3.33 X 10-5M)/
(283-0) s
= 1.17 X -7M/s
The calculations for value of K at different temperatures were:
The K value is given by;
Rate = K [I-]
[BrO-] [H+] 2
For run #1:
2.97 X 10-7M/s= K
[0.01] [0.04] [0.1]2
K = (2.97 X 10-7M/s)/ ([0.01]
[0.04] [0.1]2)
= 7.425 X 10-2
For run# 2:
= (4.97 X
10-7M/s)/ ([0.01][0.04][0.1]2)
K =
1.2425 X 10-1
For run#3:
= (9.25 X 10-7M/s)/
([0.01] [0.04] [0.1]2)
K =
2.3125 X 10-1
For run #4:
= (1.17 X -7M/s)/
([0.01][0.04][0.1]2)
K = 2.925 X 10-2
Table for values of T (K),
1/T (K-1), Rate (M/s), K and InK
Data Analysis
Graph of lnk (y-axis)
versus 1/T (x-axis, K-1). The plot of InK versus 1/T gave a straight line graph with
equation line of y= -5607.4x + 16.28 and with R2 = 0.9992. The slope
of the graph represented -Ea/R, which was the activation energy of the
reaction.
Fig1: Graph of ln K versus
1/T (K-1)
Using the equation of the
graph y = y= -5607.4x + 16.28, the activation energy of the reactions was
calculated as follows:
By taking y = mx + C, where
m is the slope of the graph and it is equivalent to -Ea/R. Hence m =
-5607.4 and m = -Ea/R, R= 8.314J/ (Kmole).
-5607.4 = -Ea/8.314J/Kmole;
-Ea =
(-5607.4)/ (8.314J/Kmole);
Ea =
674.4527J/Kmole.
Amount of time, reaction
needed at 60°C.
Using the equation InK (T2)
– InK (T1) = -Ea/R (1/T2 -1/T1), and by taking T1= 43.5°C and T2=
60°C, the value of K would be:
LnK (333) - lnK (316.5) = -5607.4(-1.5655X 10-4)
LnK (333/316.5) = 0.87786
LnK (1.05213) = 0.87786
LnK = [0.87786/1.05213]
K =
e [(0.834364574]
K =
2.30335
The rate of reaction at 60C would be:
Rate = K [I-] [BrO-] [H+] 2,
but K and concentrations of reaction were known, hence rate was;
= 2.30335 [0.01] [0.04] [0.12]
= 9.2134 X 10-6M/s
The amount of time needed for the reaction at 60˚C was;
Rate
= (3.33 X 10-5 M)/dt
Dt = (9.2134 X 10-6M/s)/ (3.33 x
10-5M)
= 0.276679s
How increase in temperature affected the reactions.
Chemical reactions increase
with increase in temperature and rule of thumb held true for this
experiment. As can been seen from the data above, reaction at temperature
31.5°C and 43.5˚C, which were 12°C apart, the time taken for the reaction at
43.5°C(36 seconds) almost doubled the reaction at 31.5°C (67 seconds).
Conclusion
K is constant only for a
specific temperature. For various temperatures, K is related to Arrhenius
equation as observed in this experiment. It was evidenced that the value of K
was directly related to rate of reaction and temperature. For instance, at
43.5°C the K value was 2.3125 X 10-1 while at 10.2°C it was 2.925 X
10-2 .The time taken at 43.5°C was less as compared to time taken at
10.2°C. This indicated that the increase in temperature increased both the K
value and the rate of reaction. On the other hand, activation energy was
independently related to the rate of reaction and the temperature. Increase in
temperature lowered the activation energy while increased the rate of reaction.








conscrysse_zo Jose Hawkins https://marketplace.visualstudio.com/items?itemName=7mifibpeke.Descargar-Dee-6--Dice-Defenders-gratuita-2022
ReplyDeletegeolocyzo
vijusWcer-na Angela Kutzler https://www.focusperformancecoachhr.com/profile/phyllypaariella/profile
ReplyDeleteboitecadre
Ncenlied_yu Todd Wheeler download
ReplyDeletefasttisuxy