Graph of Temperature Versus Equilibrium(Kq) for Ammonia Production
Ammonia is synthesized
from nitrogen gas and hydrogen gas in the Haber Process . The equation for its
production is given by;
The forward reaction is
exothermic, so, according to the Le Chatelier's Principle low temperature will
favor forward reaction. But Lower temperatures decrease the reaction rates and
for maximum yield of ammonia, temperatures between 380 and 450-degree
centigrade are used.
Recommendation: Writelearnearn
When you are provided
with the following data and you are required to plot a graph of temperature versus
equilibrium. Describe the shape of the graph for ammonia production. What can you predict from the graph?
From the graph, as the
temperature increases, the equilibrium drops abruptly according to the Van’t
Hoff Equation. Van’t Hoff equation is an equation that shows the relationship
among the temperature (T), equilibrium constant (Kq) changes and particular
change in standard enthalpy.
The catalyst used in
the production of ammonia gives maximum yield when the temperature (at least
400-degree centigrade) is applied. In the graph, equilibrium constant increases
as the temperature decreases. To produce the maximum amount of ammonia other parameters
(pressure and product removal) must be considered.
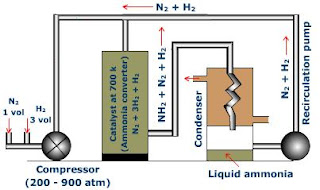
Pressure
Increasing the amount
of pressure favors the forward reaction. In the equation, 4 moles of reactants
are consumed to yield two moles of products. Pressures between 200-250
atmospheres are usually applied for maximum production. Even though, maintaining high pressure is
expensive and dangerous, working at 200 atm ensure the process is safe and
economical. The high amount of energy applied in running pumps and compressors make the process to produce 15% of ammonia in one pass.
Recommendation: Iwriter
Product
removal
Removing ammonia from the system increases its
production. Ammonia is removed from the gaseous equilibrium mixture coming out
from the reaction vessel. The hot gaseous mixture is cooled promptly to enable
ammonia to condense and to be removed in liquid form. The unreacted gasses (nitrogen
and hydrogen) are recycled in the process.






Many thanks for sharing such incredible knowledge. It's really good for your Website.
ReplyDeleteThe info on your website inspires me greatly. This website I'm bookmarked this. Maintain it and thanks again.
I'm really impressed with your writing skills, as smart as the structure of your we
GraphPad Prism Crack
I'm really impressed with your writing skills, as smart as the structure of your weblog. you may also check my website justmycrack.com.
ReplyDeleteGraphPad Prism crack
Amazing blog! I like the way you explained such information about this post to us. And a blog beneficial for us this website:
ReplyDeleteGraphPad Crystal Crack
iSkysoft iTube Studio Crack
Corel PaintShop Pro crack
CrossOver Linux crack
Thank you for sharing this information..
ReplyDeleteGraphPad Prism Crack
Nice Post, Thanks for sharing. I have used GraphPad Prism Crack Version for a long time, it's amazing. You can also other software free download from cracksilo.org
ReplyDelete<a href="https://cracksilo.org/GraphPad Prism Crack Free Download>GraphPad Prism Crack</a>
Wow, amazing block structure! How long
ReplyDeleteHave you written a blog before? Working on a blog seems easy.
The overview of your website is pretty good, not to mention what it does.
In the content!
crackpedia.net
GraphPad Prism crack
I am very impressed with your post because this post is very beneficial for me and provide a new knowledge.
ReplyDeleteSandboxie Crack
Vmware Workstation Pro Crack
GraphPad Prism Crack
Good post! We are linking to this great post on our website. Keep up the good writing. Thanks for sharing.
ReplyDeleteMediaMonkey Gold
GraphPad Prism Software
Cubase Pro
Good post! We are linking to this great post on our website. Keep up the good writing. Thanks for sharing.
ReplyDeleteNero Burning ROM Crack
GraphPad Prism Crack
EasyWorship Crack
Good post! We are linking to this great post on our website. Keep up the good writing. Thanks for sharing.
ReplyDeleteMediaMonkey Gold Crack
GraphPad Prism Software Crack
Cubase Pro 13 Crack
Microsoft Safety Scanner Crack appears to be a spyware discovery as well as elimination developer. Then cyber security analyzer develops into build.
ReplyDeleteMicrosoft Safety Scanner Crack
Microsoft Office 2010 Crack derives through an app called Microsoft Plan 2010, which permits the consumer to generate, change also portion Microsoft plans folders.
ReplyDeleteMicrosoft Office 2010 Crack